-40%
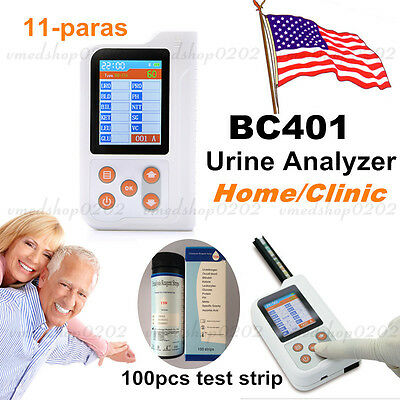
CONTEC Portable Urine Analyzer+ Printer 11 parameter with Test strip 2.8" LCD CE
$ 316.27
- Description
- Size Guide
Description
CONTEC Portable Urine Analyzer+ Printer,11ParameterIntroductions
BC400 Urine Analyzer is a high-precision, intellectual instrument which is researched and developed basing on modern optics, electronics, computer science and other advanced technologies for clinical inspection of urine. GLU, BIL, SG, KET, BLD, PRO, URO, NIT, LEU, VC and PH in urine can be tested by using it with special test strips. It can be widely used in various medical and health departments as one of the main clinical laboratory instruments.
Features
1)High-luminance and white LED, features in long life and good stability.
2)Large LCD screen, high luminance, abundant contents display, optional languages: Chinese and English.
3)User-friendly interface.
4)Optional units: international unit, conventional unit and symbol system.
5)Three working mode: one-step/slow/fast testing mode, suitable for different user group.
6)Monitoring the whole test process, auto-character and audible prompt.
7)Be compatible with 8, 10 and 11-parameter test strip.
8)Standard RS232 interface and interface for data communication.
9)Built-in thermal printer.
Performance
1)Test items: GLU, BIL, SG, KET, BLD, PRO, URO, NIT, LEU, VC and PH.
2)Test principle: RGB tricolor
3)Repeatability: CV≤1%
4)Stability: CV≤1%
5)Display: 2.8" color LCD
6)Working mode: one-step/ slow/ fast testing mode
7)Test speed: 120tests/ hour or 60 tests/hour
8)Data storage: Storage of 1000 sample data, which can be queried by test date and sample number
9)Printer: built-in high speed thermal printer
10)Interface: Standard RS-232 two-way communication interface
11)Power supply: switching power supply, 100~240V, 50/60Hz
Accessories
1)Power cable
2)Printing paper
3)User Manual
4)Test strip
Physical characteristic
Dimension: 240mm(L)×220mm(W)×130mm(H)
Weight: about 1.8Kg.
Buy safe products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE,TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved
International Buyer--Please note:
Import duties,Taxes are not included in the item price.These charges are buyers' responsibility.